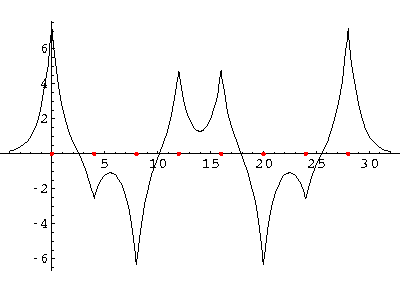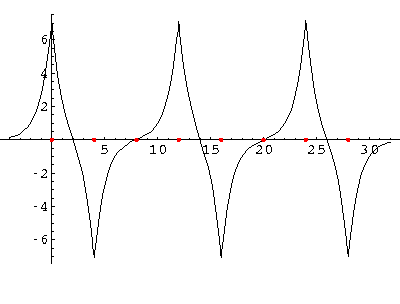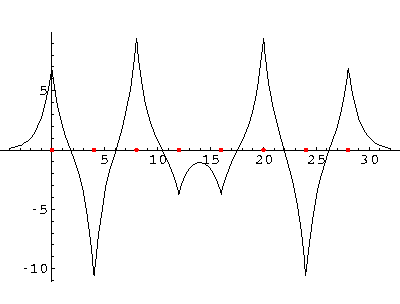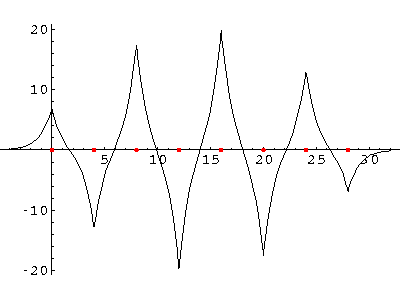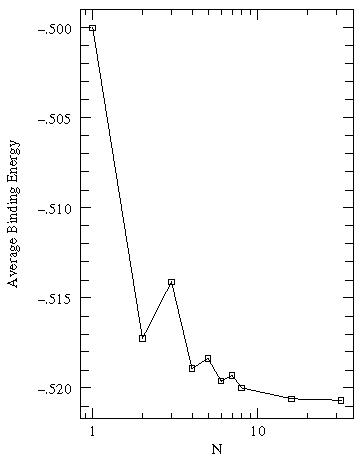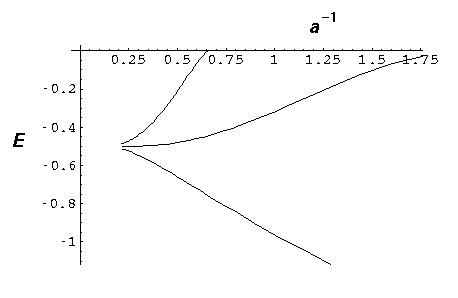
We can solve for the energy eigenvalues for three delta functions each separated by a. Here are the results:

On the lhs (large a, strong potentials, little wavefunction overlap) we have 3 nearly degenerate energies near the isolated well energy of -½. On the rhs (small a, weak potentials, large wavefunction overlap) we see those levels separate. for a=3/2 we loose one, for a=1/2 we loose the other. For zero a we have a unified well with a single bound state: E=-9/2
Now add a fourth "nucleus":
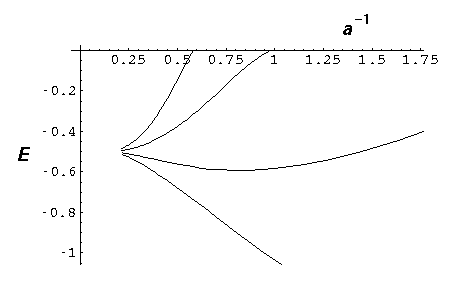
Now double that to 8 "nuclei":
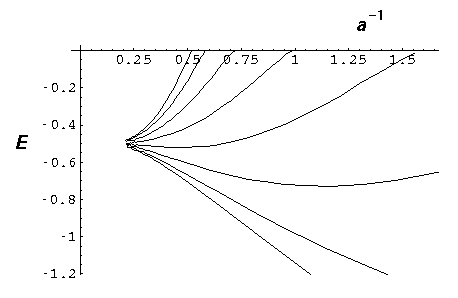
We now focus on just the energy levels for a=4 and vary the number of atoms: N=8,16,32:
Notice that as we add more atoms, we do not affect the range of allowed energy; instead we just produce more states within the same energy band. If we had N nuclei, we'd have N states in the band. Notice that within this energy band the density of states is not uniform: there are more states per energy bin at the bottom and top of the band than in the middle.
The most important point is that all of the wavefunctions are spread over all of the atoms. There is no localized chemical bond between just two atoms--every wavefunction samples the entire molecule. Approximately speaking the wavefunctions look like the symmetric sum of the solitary wavefunctions amplitude modulated (i.e., multiplied) by harmonics of a sine wave that fits the molecule. Here are the wavefunctions for N=8, with the ground state presented first. (The red dots are the delta functions.)