Falling p.6
Seeing quantum mechanical falling
Classical mechanics (and most people above age 3)
have the notion of a particle's position. Quantum mechanics
says that a particle doesn't have a position, and says it applies to all
objects: big (where it replaces classical mechanics) and small
(where classical mechanics wasn't working too well).
Now unless we've all been living in
a dream world since age 3, QM must have something that corresponds
to our very real impression that objects have position. QM finds
that "normal" objects under "normal" conditions have a range of
possible positions that is so small, that for most purposes we
can take the center of the range of possible positions as
"the" position. For example if we wanted to localize a bacteria
cell (1 µm) to an accuracy of 1/10 its diameter,
the resulting uncertainty in speed would be: 10-12 m/s,
for a Bucky Ball (C60): .04 m/s, while for a helium atom:
800 m/s (which is comparable to its normal speed at room
temperature). Thus for most any object bigger than a small molecule,
the range of possible positions required by quantum mechanics,
is so small, that usually no harm results for just saying
that the particle is in the middle of the QM range. (This
statement is hedged because physicists work hard to make macroscopic
systems in which quantum mechanics is important; but these
rare beasts are rarely found outside of the lab.)
Now it turns out that if we use the information that the
particle is approximately at a particular location, we must
give up the idea that the particle has a definite energy.
The resulting probability density then does depend on time:
the middle of the probability density (which in the above
we connect with the idea of "a" position) is seen to move much
as classical mechanics says its mythical point particles
would move.
Let's start by seeing how this would all work classically:
we say the particle starts approximately at z0
but with some uncertainty: 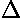 z0.
We release the particle on average with zero initial velocity,
but because of Heisenberg uncertainty relation, there must
be some spread of velocity
z0.
We release the particle on average with zero initial velocity,
but because of Heisenberg uncertainty relation, there must
be some spread of velocity 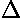 v0,
which is sometime up and sometimes down. If we knew exactly
where the particle was starting from and its speed, we'd know
exactly where it was:
v0,
which is sometime up and sometimes down. If we knew exactly
where the particle was starting from and its speed, we'd know
exactly where it was:
z(t)=z0+v0t+½at2:
But because of the initial uncertainty in the velocity, there is growing
uncertainty in position:
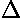 z(t)=
[(
z(t)=
[(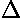 z0)2+
(
z0)2+
( v0t)2]½
v0t)2]½
Thus the packet of probability is becoming wider as the central
value of the probability follows the usual classical relation:
z(t)=z0+½at2
In our dimensionless variables this reads:
z'(t')=z'0-t'2
Our particular application will have z'0=121.5,
 z'0=0.873,
z'0=0.873,  v'0=1.50.
v'0=1.50.
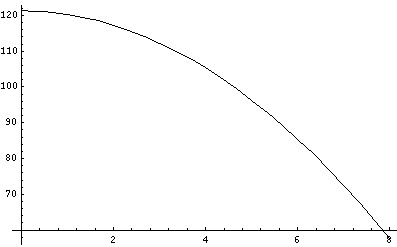
Here is the behavior of the classical probability distributions:
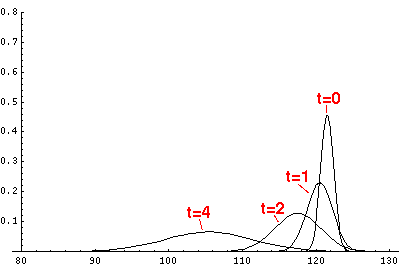
Here are the quantum mechanical results for t'=0,1,2,3,4
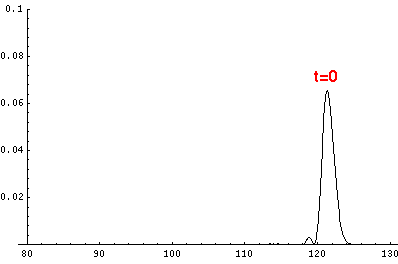
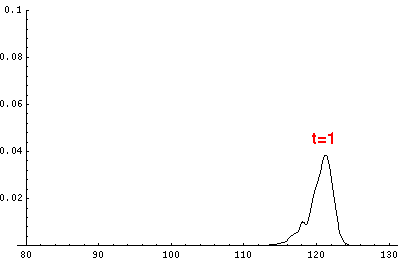
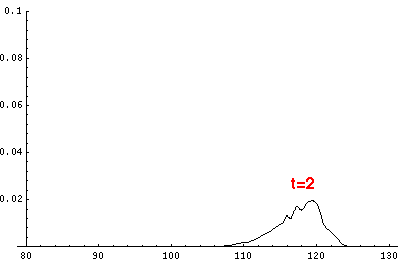
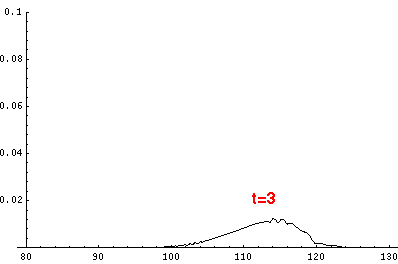
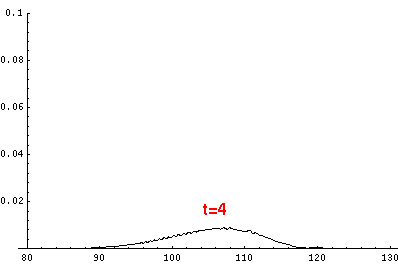
The last plot shows the average position of the wavefunction vs time. All of the
above look much like the classical result. Note that the "broadening" of the
wavefunction is simply the result of our uncertainty in the initial velocity.
Since the motion is periodic, one period later the particle must be back
where it started, so the wavefunction "narrows" on the upward bounce,
and looks like the below one period (t'=22) later:
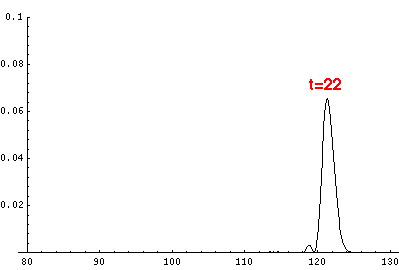
For reasons that should be clear after the next page, over several periods
the wave packet will broaden out and eventually the probability will be
distributed throughout (and a bit beyond) the classically allowed region.
Here is some data on the location of the particle:
| Time | Height
|
|---|
| 0 | 121.242
|
| 1 | 120.245
|
| 2 | 117.257
|
| 4 | 105.309
|
| 6 | 85.411
|
| 8 | 57.603
|
Next
 z0.
We release the particle on average with zero initial velocity,
but because of Heisenberg uncertainty relation, there must
be some spread of velocity
z0.
We release the particle on average with zero initial velocity,
but because of Heisenberg uncertainty relation, there must
be some spread of velocity 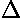 v0,
which is sometime up and sometimes down. If we knew exactly
where the particle was starting from and its speed, we'd know
exactly where it was:
v0,
which is sometime up and sometimes down. If we knew exactly
where the particle was starting from and its speed, we'd know
exactly where it was:







