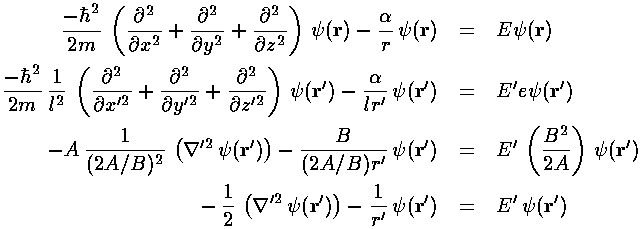
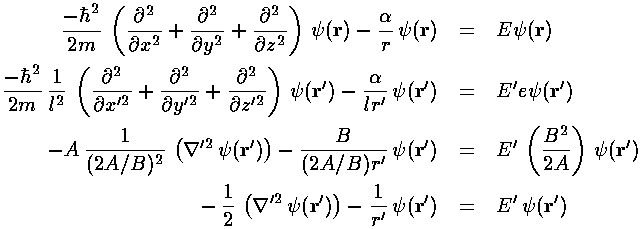
Since the problem is rotationally symmetric rather
than square-symmetric, it makes more sense to use a coordinate
system that reflects the symmetry in the problem. Hence,
I'd like to solve this problem is spherical coordinates
(r'- -
- ).
).
Changing coordinates changes the Schrödinger's equation to:
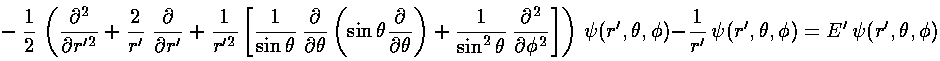
The stuff in the square brackets (with the overall minus sign) is the (dimensionless) angular momentum squared operator: L'2. The eigenfunctions of this operator are degenerate, so there is a choice to make in defining the basis states. In physics folks conventionally use the Ylm, which are simultaneously eigenfunctions of both L'2 and L'z:
L'2 Ylm = l(l+1) Ylm
L'z Ylm = m Ylm
Chemists not uncommonly use a different basis which has the advantage that the functions are real, but the disadvantage that they do not display spherical symmetry.
The probability density Ylm*Ylm
is independent of  ("cylindrically symmetric" for a cylinder
whose axis is aligned with the z axis) so we only need to concern
ourselves with their dependence on
("cylindrically symmetric" for a cylinder
whose axis is aligned with the z axis) so we only need to concern
ourselves with their dependence on 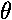 . As you would expect
if the angular momentum is aligned with the z axis (so m=l)
the probability should mostly be in the x-y plane, i.e.,
. As you would expect
if the angular momentum is aligned with the z axis (so m=l)
the probability should mostly be in the x-y plane, i.e.,
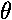 =
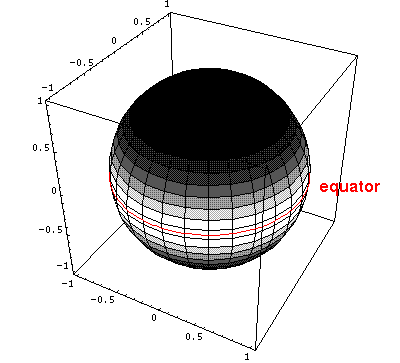
=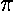 /2=90°. Below is
a plot of the probability density for l=m=5.
/2=90°. Below is
a plot of the probability density for l=m=5.
Here is the corresponding plot just as a function of 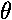 .
.
On the other hand, if m=0, the angular momentum vector must be
in the x-y plane and the particle must be moving perpendicular
to the angular momentum vector and hence in part in the
z direction. For example, if L~i
(i.e., the angular momentum vector is in the x direction), we expect
motion to be in the y-z plane. The m=0 solutions
are essentially superpositions of all possible orientations of L
in the x-y plane. What survives the superposition is:
(1) a big amplitude on the z-axis where all orientations
contribute, (2) oscillation
(motion) in 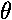 and (3) uniform distribution in
and (3) uniform distribution in
 .
Below is
a plot of the probability density for l=5, m=0.
.
Below is
a plot of the probability density for l=5, m=0.
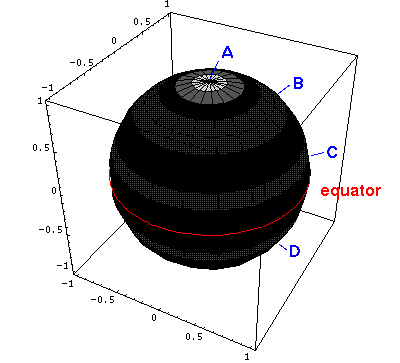
Here is the corresponding plot just as a function of 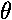 .
.
(Here is a bit more information about the Ylm and other choices.)
Seeking a solution where the dependence on r' factors
with all the 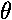 ,
, dependence in Ylm ("separation of variables"),
i.e.,
dependence in Ylm ("separation of variables"),
i.e.,  =R(r')Ylm
we find:
=R(r')Ylm
we find:
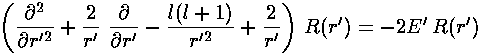
As usual we have to work a bit to solve for the r' wavefunction.
Start by seeing how the equation must work for large r'.
Most of the terms vanish for large r'.
Only the first term on the left has a chance of equaling
the term on the right. Ignoring the (small) terms, we seek to find
the form for  that solves the differential
equation for large r' (remember E'<0 so -E'=|E'|)
that solves the differential
equation for large r' (remember E'<0 so -E'=|E'|)
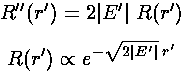
Note that for large r',  is a function
of |E'|½r'. We take this as a hint
to use a variable proportional to |E'|½r'
everywhere. Thus we define
is a function
of |E'|½r'. We take this as a hint
to use a variable proportional to |E'|½r'
everywhere. Thus we define
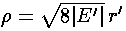
and rewrite our r' differential equation in terms of
 . To do this divide the above r' differential equation
by 8|E'|, yielding:
. To do this divide the above r' differential equation
by 8|E'|, yielding:
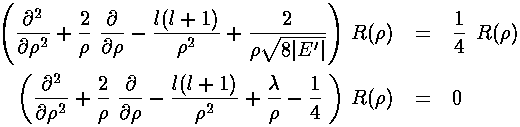
Note that we have eliminated E' in favor of a new constant
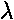
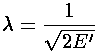
For small  only the derivative terms have
a chance to match the ever growing
l(l+1)/
only the derivative terms have
a chance to match the ever growing
l(l+1)/ 2 term.
If we try a power-law solution
2 term.
If we try a power-law solution  n
n
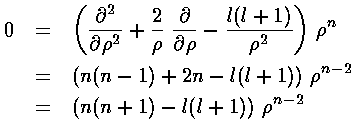
we find n=l or n=-(l+1); the former
is not singular as  nears 0
nears 0
Factoring out all the required behavior for large and
small  , we hope to find a simple function
(polynomial with luck) H(
, we hope to find a simple function
(polynomial with luck) H( ) that contains the
behavior for intermediate
) that contains the
behavior for intermediate  .
.
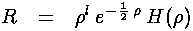
Now the work begins: we need to express all the derivatives of R in the differential equation in terms of derivatives of H
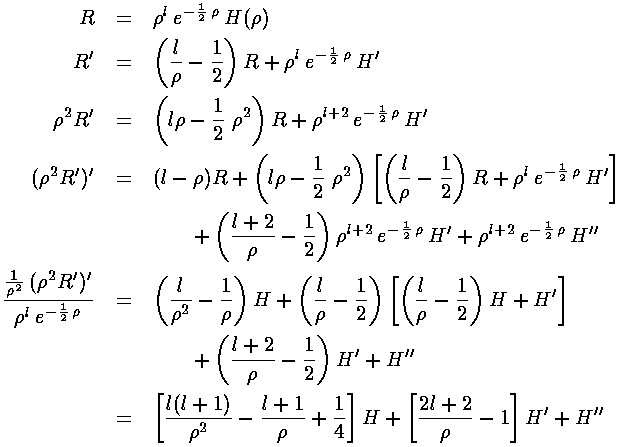
Using these derivatives in the R differential equation gives use what we hope is an easier to solve differential equation for H:
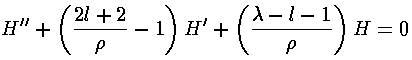
If we now try to write H as a polynomial:
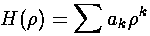
we can plug the polynomial form into the differential equation:
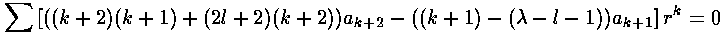
The result is a two term recursion relation (i.e., the result has just two as so, for example, given a0 we can calculate a1, from which we can calculate a2, etc. until we're done.)
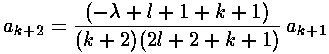
It is convenient to shift the recursion relation down
one (i.e., k k-1):
k-1):
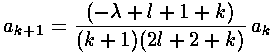
It turns out that the polynomial must end if the
wavefunction is to be normalizable. One can show that the non-terminating
sum gets at least as big as exp(+ ), so
), so
 instead of going to zero for large
instead of going to zero for large  gets
big like: exp(+
gets
big like: exp(+ /2). The only way the sum can end is if
the numerator of the recursion relation is zero, i.e., if
/2). The only way the sum can end is if
the numerator of the recursion relation is zero, i.e., if 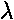 is an integer n (which we call the principal quantum number).
nr (called the radial quantum number) is the highest
power in the H polynomial.
is an integer n (which we call the principal quantum number).
nr (called the radial quantum number) is the highest
power in the H polynomial.
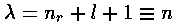
Now that we know 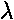 , we can find E:
, we can find E:
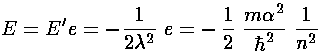
In the the case of an atom, we can express the force constant
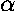 in terms the the number of protons in the
nucleus (Z) and the charge on the nucleus (in cgs units)
(e):
in terms the the number of protons in the
nucleus (Z) and the charge on the nucleus (in cgs units)
(e): 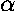 =Ze2. So:
=Ze2. So:
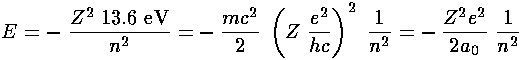
We also find a simple relationship between  and r':
and r':
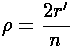
Note that if we had tried for a polynomial solution for R
itself (rather than factoring out the
 lexp(-
lexp(- /2) first),
we would get an (unsolvable) three term recursion relation.
/2) first),
we would get an (unsolvable) three term recursion relation.
The polynomials we have been calling H are Laguerre polynomials (see for example, Abramowitz & Stegun, §22 p. 771 or Szegö Ch.V).
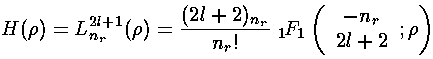
| n | E' |
|---|---|
| 1 | -0.500 |
| 2 | -0.125 |
| 3 | -0.056 |
| 4 | -0.031 |
| 5 | -0.020 |
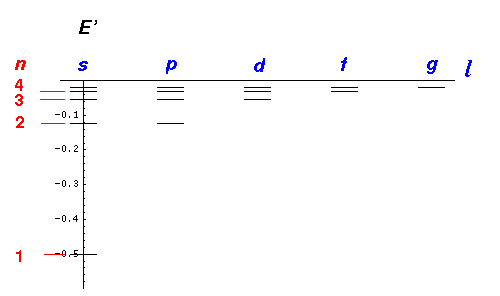
| n | Included States | Total Degeneracy |
|---|---|---|
| 1 | 1s | 1 |
| 2 | 2s,2p | 4=1+3 |
| 3 | 3s,3p,3d | 9=1+3+5 |
| 4 | 4s,4p,4d,4f | 16=1+3+5+7 |
| 5 | 5s,5p,5d,5f,5g | 25=1+3+5+7+9 |
Note that atomic states are labeled by the principal quantum number n, thus there is no 1d state; the first d state is 3d.
Note that the degeneracy count of a particular (n,l) state described above [e.g., n=5, l=3 is 5f] is the result of the different values of m allowed with that l value: m may range in integer steps from -l to l, a total of 2l+1 possible values. Thus the degeneracy of the 5f state is listed as 7=2l+1 for l=3 in the equation: 25=1+3+5+7+9. The fact that the energy does not depend on m (the orientation of the angular momentum [classically the orbit's orientation in space]) but rather on l (the total amount of angular momentum) is the result of spherical symmetry.
The other piece of this degeneracy -- the fact that the energy depends on
the sum of nr and l through:
n = nr + l +1
so 5s,5p,5d,5f,5g
[i.e., (nr,l)= (4,0), (3,1), (2,2), (1,3), (0,4) ]
all have the same energy -- is a more complicated story. Here I simply mention the
name: Lenz vector (a.k.a. Laplace-Runge-Lenz vector). This degeneracy means that
there are five terms to add in the equation: 25=1+3+5+7+9